This paper was completed by a researcher at the Institute of Petrochemical Technology of Lanzhou University of Technology to discuss the paper on the synthesis of multi-stage pore CuAPO-5 molecular sieve by ion thermal method, which was published in the important journal .
CuAPO-5 molecular sieve with multi-stage pore structure was rapidly synthesized by microwave irradiation using succinic acid, choline chloride and tetraethylammonium bromide ternary eutectic as solvent and template. The ratio of the mass ratio of P2O5/Al2O3, HF/Al2O3 and CuO/Al2O3, and the influence of aluminum source and copper source on the synthesis of CuAPO-5 molecular sieve were systematically investigated. The crystallinity, morphology and pore structure of the synthesized products were characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and N2 physical adsorption and desorption. It was found by SEM that under certain conditions, a CuAPO-5 molecular sieve having a hexagonal nanodisk-like special morphology can be obtained. The physical adsorption, desorption, SEM and TEM analysis of N2 showed that the molecular sieve was a multi-stage aluminum phosphate molecular sieve material with micropores and mesopores.

Fig.1/3↑

Fig.2/3↑
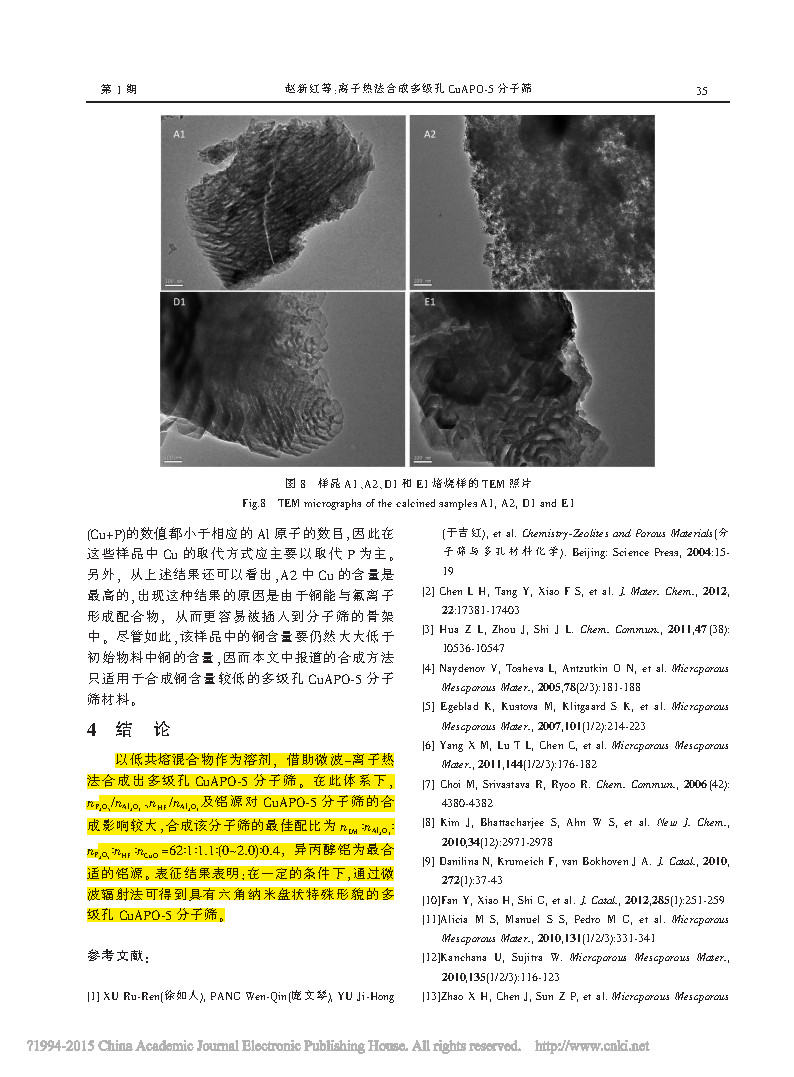
Fig.3/3↑
The multi-stage pore CuAPO-5 molecular sieve was synthesized by microwave-ion thermal method using a eutectic mixture as a solvent. Under this system, nP 2O5/nAl2O3, nHF /nAl2O3 and aluminum source have great influence on the synthesis of CuAPO-5 molecular sieve. The optimal ratio of synthesis of this molecular sieve is nEM:nAl2O3:nP 2O5:nHF:nCuO =62:1 : 1.1: (0~2.0): 0.4, aluminum isopropoxide is the most suitable aluminum source. The characterization results show that under certain conditions, the multi-stage pore CuAPO-5 molecular sieve with hexagonal nanodisk shape can be obtained by microwave irradiation.
A typical process for the synthesis of CuAPO-5 molecular sieves is as follows: first a eutectic mixture of succinic acid, choline chloride and tetraethylammonium bromide in a ratio of 8:8:1. EM), copper citrate and aluminum isopropoxide were uniformly mixed by grinding, placed in a three-well flask, and then phosphoric acid and hydrofluoric acid were added. The ratio of the amount of the substance obtained in the final mixture was: nEM:nP2O5:nAl2O3:nHF:nCuO=62:(1.1~2.2):1.0:(0.0~2.0):0.4. The mixture was heated with an electric heating sleeve to be melted, and then placed in a microwave synthesizer to be heated to 180 ° C, and crystallization was carried out at this temperature for 1 hour under normal stirring conditions.
