This research was completed by a researcher from the Key Laboratory of Comprehensive Utilization of Tailings Resources in Shaanxi Province. The paper discusses the preparation of active zinc oxide by leaching zinc oxide from ammonia, and published it in the important journal .
In order to realize the reuse of zinc resources in the zinc slag waste residue, the zinc oxide dust generated in the zinc smelting process of the Shangluo zinc smelting plant is used as the raw material, and the granules with uniform particle size distribution are obtained by the ammonia leaching-microwave evaporation ammonia-fire method. Active zinc oxide. The leaching process of zinc and the pyrolysis process of zinc oxide precursor were studied, and the structure and phase of the product were characterized by TG/DTA, XRD and SEM. Studies have shown that the total ammonia concentration during ammonia leaching is 8mol / L, pH is 10.0, liquid-solid ratio is 4:1, leaching temperature is 40 °C, the leaching rate of zinc is up to 92.05%. After the leachate was cleaned and decontaminated by two stages, the precursor basic zinc carbonate was prepared by steaming ammonia at 80 °C for 25 min. The average particle size of about 3 μ m, hexagonal spheroidal active zinc oxide was obtained by calcination at 400 ° C for 120 min. This method has low requirements on equipment, low production cost, short process flow and strong practicability.

Fig.1/3↑

Fig.2/3↑
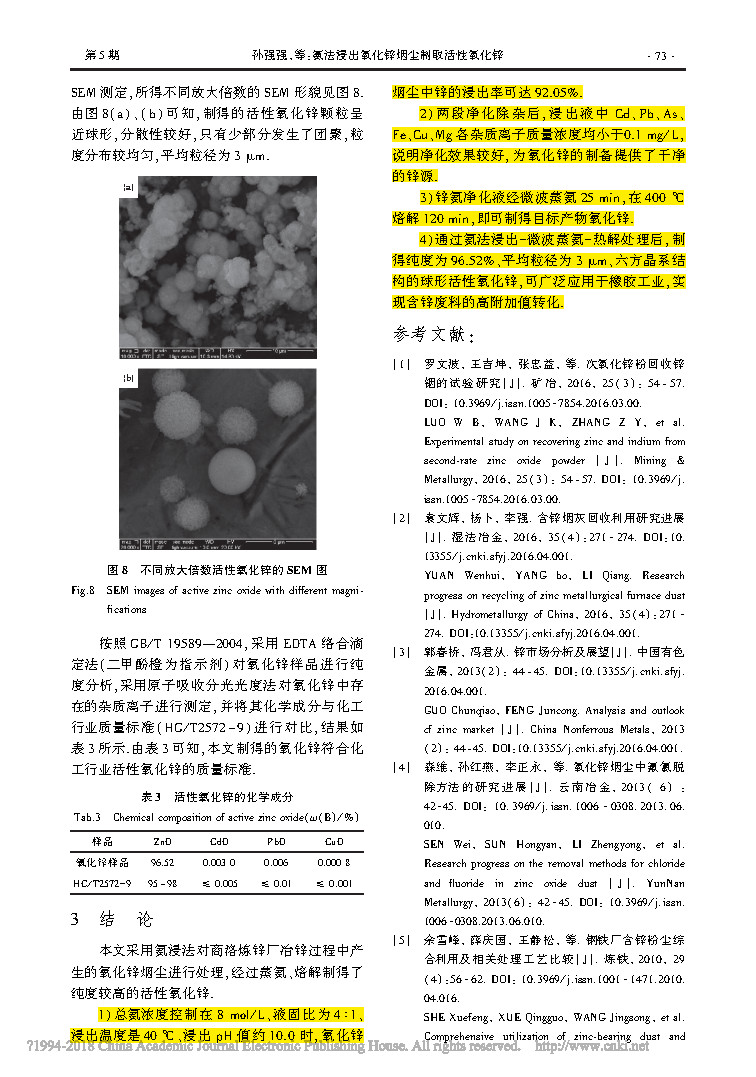
Fig.3/3↑
1) When the total ammonia concentration is controlled at 8mol/L, the liquid-solid ratio is 4:1, the leaching temperature is 40°C, and the leaching pH is about 10.0, the leaching rate of zinc in zinc oxide soot can reach 92.05%. 2) After purification and impurity removal in two stages, the mass concentration of impurity ions of Cd, Pb, As, Fe, Cu and Mg in the leachate is less than 0.1mg/L, indicating that the purification effect is better, which provides a clean preparation for zinc oxide. Zinc source. 3) The zinc ammonia purification solution is subjected to microwave evaporation for 25 min and baked at 400 °C for 120 min to obtain the target product zinc oxide. 4) After leaching by ammonia method - microwave evaporation of ammonia - pyrolysis treatment, spherical active zinc oxide having a purity of 96.52% and an average particle diameter of 3 & mu; m and a hexagonal crystal structure can be obtained, which can be widely used in the rubber industry. Achieve high value-added conversion of zinc-containing waste.
Weigh 3g of zinc oxide soot into a 100mL round bottom flask, add 40mL distilled water, 12mL ammonia-hydrogen ammonia ammonia solution (total ammonia concentration is 8 mol/L), at 40 °C, pH value is 10.0, liquid solid The reaction was carried out for 1.5 h at a stirring speed of 400 r/min, filtered under reduced pressure, and the filter cake was washed three times with distilled water, and the filtrate was transferred to a 100 mL volumetric flask to make a volume. Take 20 mL of zinc ammonia leaching solution, determine the concentration of zinc ion by atomic absorption spectrophotometry and calculate the leaching rate of zinc.
