
Author Affilications:School of Mineral Processing and Bioengineering, Central South University
Abstract: In order to address the dual challenges of environmental pollution and sustainable energy systems, this study investigates a novel COsequest technology through steel slag, a by-product of steel production. It introduces an ultrasonic-enhanced leaching process that uses CHCOOH to extract calcium and magnesium from steel slag, thereby facilitating the synthesis of CaCOwhiskers. The results showed that the selective leaching rate of Ca + Mg was significantly increased by increasing the ultrasonic power, improving solid-liquid consistency, increasing the initial reaction temperature, and using lower concentrations of CHCOOH. Kinetic analysis under ultrasonic conditions showed a low activation energy of 11.45 kJ•mol, indicating the presence of surface-controlled reaction kinetics during ultrasonic leaching. Eventually, high-purity CaCOwhiskers were synthesized under carefully controlled conditions: using 400 W of ultrasonic power, maintaining a reaction temperature of 70 C, and performing the procedure for 15 minutes with an initial solution pH of 9.2. This innovative approach not only helps to reduce CO2 emissions, but also promotes the reuse of steel slag, addresses environmental issues and supports a sustainable energy system. The report highlights the potential to integrate sustainable practices into the steel industry, in line with global efforts to mitigate climate change and reduce pollution.
Keywords: Ultrasonic chemistry, slag fixation, selective leaching, CaCOwhisker
Introduction: In the global carbon emission industry, the steel and cement industries occupy an important position. It is important to note that the steel industry is characterized by its long main processes, which are clearly characterized by low concentrations and large emissions in the COemission of its various sub-processes. The use of traditional carbon capture and storage (CCS) technologies can significantly increase the expenditure of steel industry institutions. Implementing chemical conversions to harness CO can be a costly barrier to real reduction in CO emissions, impacting economic and environmental benefits. As shown in Figure 1, CO mineralization is the only way for CO to be utilized, and is characterized by a negative variation in Gibbs free energy (ΔG < 0). This suggests that mineralization reactions occur naturally and do not require additional energy inputs. Therefore, the application of mineralization to utilize COi is considered an excellent way to quickly address the problem of low CO emissions. Repurposing solid waste for sequestering CO emissions is a prominent and viable solution. [1,2]
According to rough estimates, 0.13 to 0.2 metric tons of steel slag are produced for every tonne of steel produced domestically. As a result, steelmakers have amassed more than 1.45 billion metric tons of inventory by adopting an expanded steelmaking process. [4] In the field of slag management and utilization, the main strategy adopted by most companies involves the use of accumulation disposal methods. Prolonged storage of steel slag can lead to soil and groundwater contamination, increasing the risk of airborne dust affecting air quality and wasting valuable land resources. As a result, the adjacent terrestrial and aquatic ecosystems are eventually polluted, posing a major threat to environmental security. The composition of steel slag is very broad and mainly includes FeO, CaO, SiO, and MgO. CaO and MgO are the main basic components in steel slag. Each metric ton of steel slag can sequester 385-600 kg of carbon monoxide, highlighting its excellent carbon sequestration potential. [5,6] Through their reaction with COin steel, slag, CaO, and MgO permanently fix CO in the process of forming CaCO and MgCO. This capability can be used to reduce CO emissions and the production of high-value carbonate compounds. It contributes to sustainable resource recovery and environmental stewardship.
The chemical composition of steel slag is very similar to that of cement clinker and constitutes a potential pozzolanic material. However, it contains large amounts of unstable f-CaO and f-MgO, which react with water to form Ca(OH) and Mg(OH), resulting in volume expansion and considerable stability challenges. The use of steel slag for CO storage effectively solves these problems while promoting the mineralized utilization of CO. At present, the pathways of CO sequestration in steel slag mainly include direct and indirect processes. The direct sealing process of steel slag is simple and clear, the procedure is concise, and the operation is simple. [7–9] However, it imposes fairly strict reaction conditions. At ambient temperature, the carbon sequestration rate in steel slag is significantly slow, and a large amount of energy needs to be consumed to increase the reaction temperature, thereby accelerating the carbon solidification rate.
Indirect sequestration in steel slag refers to the leaching of calcium and magnesium minerals from slag using a leaching medium. Subsequently, these dissolved alkaline substances react with the COin gas stream at pH greater than 7 to produce a stable carbonate product. [10,11] While the process has an extended procedural pathway, it is characterized by reduced energy requirements and the ability to produce high-purity CaCO products. However, there are many challenges to be addressed in the current methods of indirect storage in steel slag, including concerns about leaching media selection, quality control of CaCO products, and residue resource utilization. In the steel slag leaching process, the choice of leaching agent mainly revolves around strong acids [12–14], weak acids [15], and ammonium salt solutions. [16–18] Investigations have shown that the use of strong acids as leaching agents, such as hydrochloric acid, nitric acid, and sulfuric acid, places high demands on the acid resistance of leaching equipment. In addition, this method results in the extraction of a large number of impurity ions during the leaching process. A large amount of impurity remover is consumed during the purification process, which affects the quality of the carbonate product.
When ammonium salts are used as leaching agents, the process exhibits significant selectivity for the calcium-magnesium component in steel slag. [19,20] However, the leaching efficiency is still not ideal, and for some ammonium salts (e.g., ammonium sulfate), the leaching of steel slag can lead to the formation of calcium sulfate products. These compounds are incorporated into the leaching slag, making industrial-scale applications challenging.
The use of low-carbon atomic organic weak acids as substitutes for strong acid media to leach calcium and magnesium from steel slag is a prominent topic of discussion. Hong et al. [21] used organic acids derived from biomass waste, such as acetic acid, propionic acid, butyric acid, and valeric acid, as leaching agents to improve the extraction efficiency of calcium and magnesium elements from steel slag. Studies have shown that low-carbon atomic organic weak acids exhibit significant selectivity in leaching calcium and magnesium elements from the mineral phase. Low-carbon organic acids come from a variety of sources and are usually obtained through a biological fermentation process. Notably, they are recyclable and have great prospects in a variety of applications. [22] The presence of non-reactive solid inert minerals during leaching, in which calcium-bearing mineral phases remain uninvolved, poses a challenge to achieve high leaching efficiency in these mineral phases. Therefore, elucidating how to exploit the leaching potential of organic acids constitutes a critical and complex consideration for further exploration.
The use of Xianghu instruments in this article:

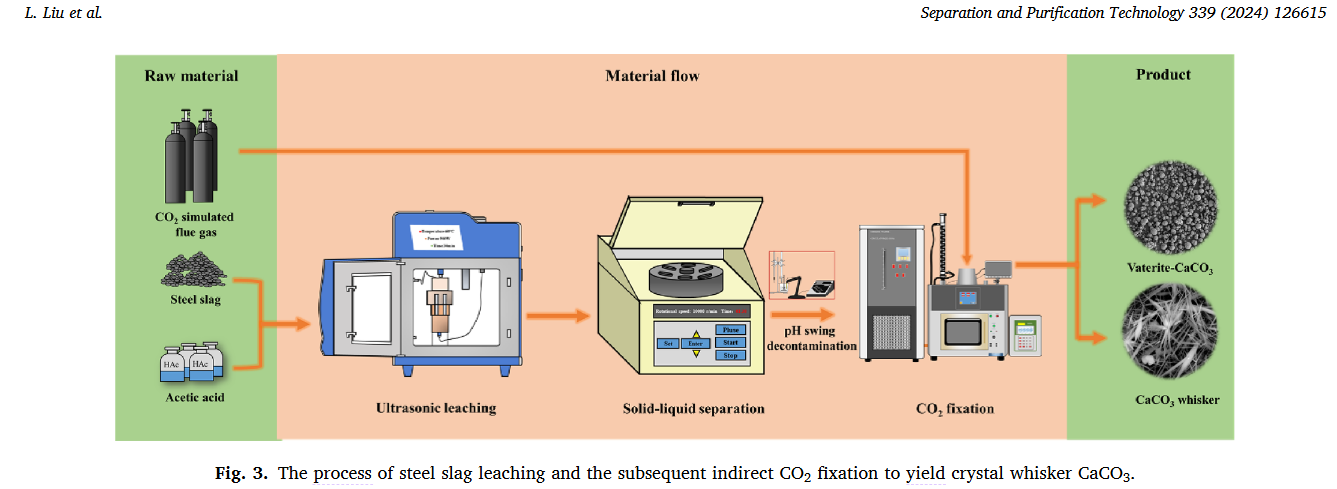
Conclusion: In this study, green CHCOOH leaching agent was used to selectively and efficiently extract calcium and magnesium from steel slag in an ultrasonic water bath reaction system. Compared with the traditional reaction field, the ultrasonic reaction field is conducive to refreshing the reaction interface of steel slag and promoting the activation of CHCOOH molecules, so ultrasonic leaching is conducive to shortening the leaching time under similar leaching efficiency. The leaching kinetics of 11.45 kJ⋅mol calcium activation energy based on the Arrhenius diagram strongly support the surface chemical control mechanism. What's more, a high selective leaching rate of Ca + Mg can be obtained at 0.5 M CHCOOH, a reaction temperature of 60 °C, a reaction time of 30 minutes, a solid-liquid ratio of 25 g/L, and an ultrasonic power of 500 W. Optimal leaching is carried out under ideal conditions, and the solution undergoes a pH fluctuation process in which Fe and Al precipitate sequentially, resulting in a calcium-rich solution with a calcium-magnesium ratio of 6 at a pH of 9.2. Finally, with a curing temperature of 70 °C, a reaction time of 15 min, and an ultrasonic reaction field of 400 W, the heterolytic leachate fixed CO to form whisker-like CaCO, demonstrating its potential in the production of COsecap and high-value CaCO.
